Academic research has looked at the roles of specific types of cells or regions of the brain in Alzheimer’s disease. But the Allen Institute and its collaborators have a bigger ambition—to draw a comprehensive map of cellular and molecular changes during the development of Alzheimer’s, hoping to identify new targets for future therapies.
The initiative, called the Seattle Alzheimer’s Disease Brain Cell Atlas (SEA-AD), has released some of its first findings at the Alzheimer’s Association International Conference.
“Because we don’t know yet which of many possible pathways are most important in Alzheimer's, we need to have the most comprehensive picture we possibly can of the related changes in brain cells,” Richard Hodes, M.D., director of the National Institutes of Health's National Institute on Aging, said in a statement. “Progress such as this means we have an increased understanding of what underlies the disease, and therefore a better hope for strategizing to effectively prevent and treat it.”
To create the atlas, scientists led by Ed Lein, Ph.D., at the Allen Institute and collaborators at the University of Washington and Kaiser Permanente Washington Research Institute have profiled more than 1.2 million neurons and other brain cells from 84 donors. The data set includes cellular and molecular information as well as microscopy images of amyloid beta and other proteins implicated in Alzheimer’s.
The donors cover different stages of Alzheimer’s, from those who aged without the neurodegenerative disease to people with severe dementia. The idea is to pinpoint the differences across various brain cell types from the samples, offering clues to targeted therapies to protect vulnerable cell populations.
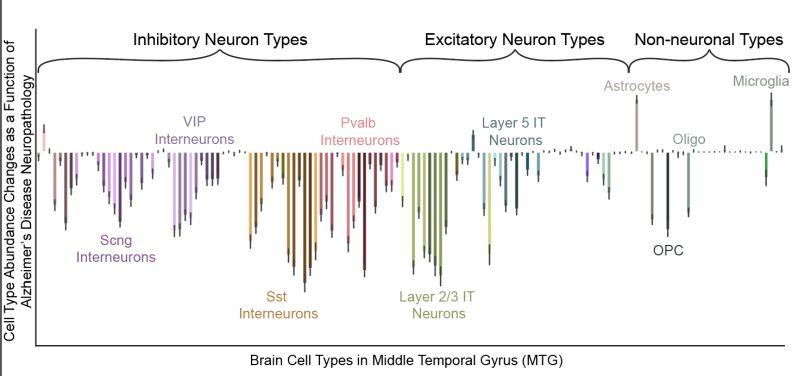
In their first analysis, the scientists focused on a region of the cortex known as the middle temporal gyrus, which is affected about midway during the progression of Alzheimer’s.
They found that some neurons that make long-distance connections within the cortex showed a different profile, suggesting the neurons may be vulnerable to or involved in Alzheimer’s. They also noticed an increase in microglia cells, a type of immune cell of the brain responsible for removing cellular debris.
Microglia cells could move to clear early amyloid beta and tau protein aggregates in the brain in the beginning stages of Alzheimer’s. But this response is viewed as a double-edged sword, as its overreaction can promote neuroinflammation and worsen the disease.
In a previous study, scientists at Massachusetts General Hospital found that interleukin-3 (IL-3) might help block neuroinflammation in Alzheimer’s because it facilitates the crosstalk between microglia and star-shaped glial cells known as astrocytes. IL-3 could reprogram microglia to increase the immune cell’s ability to clear protein aggregates in mice, that team showed.
The Allen Institute’s SEA-AD program has also been using single-cell RNA sequencing to characterize the expression patterns of genes in individual cells to help scientists investigate the underlying mechanism behind vulnerable neurons. The SEA-AD data set, now openly available to the scientific community, provides gene activity information in single cells between the healthy brains and brains from the donors.
“We want to describe trajectories of the disease as they march across brain regions and across different cell types in those brain regions, including regions affected both early and late in the disease,” Lein said. “The aim, ultimately, is to find the early, causal events that happen when the disease is still potentially reversible.”