Not long after locking down FDA clearance for a device used to collect high-volume blood samples from the comfort of a user’s own home, Tasso is once again expanding its reach—both geographically and in terms of its product portfolio.
The Seattle-based company’s latest regulatory nod came in the form of a CE mark approval in the European Union, Tasso announced Tuesday. The TassoOne Plus blood collection device successfully met the requirements of the EU’s new and somewhat cumbersome Medical Device Regulation, allowing it to be used by healthcare providers and academic and pharmaceutical researchers across the continent.
“The demand for convenient, patient-centric care is exploding, and Tasso is on a mission to bring high-quality healthcare into homes worldwide,” said CEO Ben Casavant, Ph.D. “This CE mark unlocks clinical-grade liquid blood collection for decentralized clinical trials and home healthcare within the European Union, accelerating and expanding access to care.”
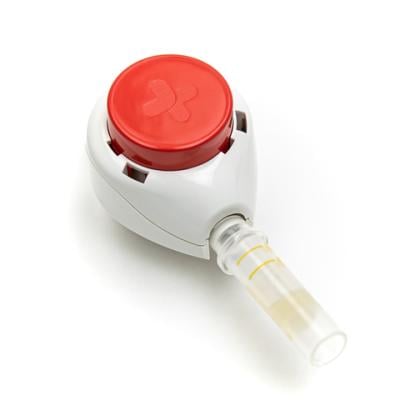
The Fierce 15 honoree’s home-use devices are designed to provide a simple and “virtually painless” way for individuals to collect their own blood samples. Users simply stick the device to their upper arm, push the big red button and wait for five minutes as the connected collection tube or pod fills with blood. Once complete, the device can be easily peeled off the arm, and the collection container is sealed and sent off for testing with the included shipping instructions and labeling.
In studies of its technology, Tasso has reported that 99% of users preferred using the pared-down device to undergoing a traditional blood draw in a clinic or even using an at-home fingerstick kit, and about 97% said they would readily repeat Tasso’s collection process.
The button-activated devices are meant to be used in remote clinical trials and telehealth services. For those users, in addition to the blood collection technology, Tasso also offers a logistics software platform to help healthcare providers and researchers order tests to patients’ homes and coordinate the samples’ submission to diagnostic labs, while tracking the progress of the test kit the entire way.
The newly cleared TassoOne Plus is able to collect clinical-grade liquid blood samples in larger volumes than those taken by many other at-home sample kits—between 250 and 500 microliters for Tasso’s devices, compared to a limit of 30 microliters for several other kits.
The CE mark arrives just a few months after the FDA cleared another high-volume device, the prescription-only Tasso+ model, in the U.S.; at the time, the company said it would begin shipping out the Tasso+ device to pharma companies, healthcare organizations and academic institutions by the end of 2022.
Tasso has also previously snagged CE mark clearance for the Tasso-M20 device. Though the M20 model uses the same push-button blood collection method as its sister devices, it collects a much smaller sample and separates it into four individual dried samples—each measuring in at 17.5 microliters—rather than leaving it as a single tube of liquid blood.