The FDA has approved its first shock absorber for the knee, with a reinforcing implant designed to serve as an option before turning to a total joint replacement.
Developed by Moximed, a 2015 Fierce Medtech Fierce 15 winner, the spring-loaded Misha Knee System connects and cushions the thigh and shin bones of people suffering from painful osteoarthritis—especially those who have not found relief from previous treatments, including surgery.
That also includes patients who may be ineligible for total knee replacement surgery or have early stages of osteoarthritis, or OA, as well as those who may be unwilling to undergo the full procedure due to their age.
“This is a milestone event for knee OA sufferers, and it’s the result of unwavering clinical research and development that spans more than 10 years,” Moximed founder and CEO Anton Clifford said in the company’s announcement.
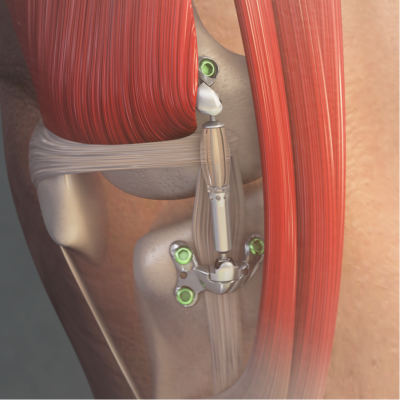
According to the company, the implant can be placed in an outpatient setting without needing to cut bones or permanently modifying the joint.
A clinical study presented last fall showed it performed better at reducing the load and forces on the knee than high tibial osteotomy, a surgical procedure that aims to realign and preserve the joint and postpone the need for a replacement.
Previous studies of an earlier version of the Misha implant showed recipients went five years without needing to progress to a knee replacement, the company said.
Moximed now plans to scale up its business as it introduces the Misha system to the U.S., after raising a total of $40 million last August through a series C venture capital round and debt financing backed by Advent Life Sciences, New Enterprise Associates, Future Fund, Vertex Healthcare, Gilde Healthcare, GBS Venture Partners, Morgenthaler Ventures and Runway Growth Capital.
The company estimates that osteoarthritis currently affects more than 32 million people in the U.S., a number forecast to grow to as many as 70 million by 2040.