Two years after submitting the latest version of its insulin pump technology for FDA approval—and three years after it was first made available in Europe—Medtronic has finally secured the agency’s signoff to begin selling the MiniMed 780G system in the U.S.
Medtronic announced the FDA approval late Friday. It allows people with Type 1 diabetes who are at least 7 years old to use the algorithm-equipped diabetes management system, which combines the MiniMed pump with Medtronic’s Guardian 4 continuous glucose monitoring sensor and SmartGuard technology for automated insulin adjustments.
Both the Guardian 4 sensor and the MiniMed pump can be used for up to seven days before requiring a hardware swap, with the latter thanks to the company’s recently launched infusion set that boasts more than double the typical life span of other insulin pump tubing.
Preorders for the system will open May 15, with the first 780G shipments expected to begin “later this summer,” per the devicemaker, which noted in the announcement that current users of the 770G system will be eligible for a free, remote software upgrade to the 780G technology.
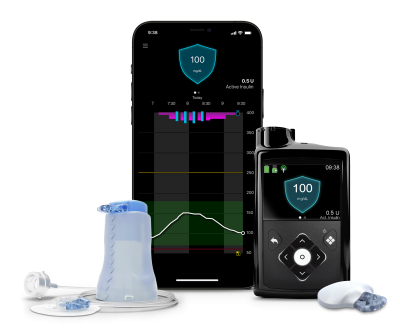
The new MiniMed system is equipped with an upgraded version of Medtronic’s SmartGuard technology. When used in “Auto Mode,” previous versions of the algorithm could take in blood sugar readings from a Guardian sensor and transmitter and then, every five minutes, automatically adjust a connected MiniMed pump’s basal insulin doses—or completely pause insulin delivery—to keep users within a healthy glucose range. When it came to bolus doses of insulin, however, those versions of the SmartGuard tech could offer only recommendations to bring down glucose levels.
Not anymore: The new and improved SmartGuard system includes an algorithm that can automatically deliver correction bolus doses if a user’s blood sugar spikes above the preset range. People with insulin-dependent diabetes typically have to calculate bolus doses at mealtimes based on the number of carbs they’re about to consume, so the upgraded algorithm could help pick up the slack if they forget to take a dose before a meal or incorrectly calculate the carb count, according to Medtronic.
“Mealtimes prove to be one of the biggest challenges for people living with type 1 diabetes, and now for the first time, the MiniMed 780G system addresses this unmet need with automatic, real-time insulin corrections,” Que Dallara, president of the company’s diabetes division, said in the announcement.
“A lot can happen to blood sugars in the span of an hour or even just a few minutes, so we’ve designed our system for real life—the algorithm adapts to the user and helps compensate for everyday challenges that are quite common around mealtimes,” Dallara said. “We built in features informed by extensive customer feedback, and we’re excited to deliver a system with ease of use at the forefront.”
Medtronic originally submitted the MiniMed 780G system for FDA approval in spring 2021, but the regulatory decision was delayed after the company spent much of last year correcting quality control issues that the agency had discovered at the headquarters of Medtronic’s diabetes business and outlined in a late 2021 warning letter.
In the meantime, the 780G technology scored regulatory clearances in dozens of other countries, and its maker continued to release study data proving the system’s mettle.
Results of the ADAPT trial that were originally published last fall, for example, showed adults saw their average blood sugar readings drop by about 1.4% after six months of using the MiniMed 780G system. That helped the amount of time they spent within the ideal glucose range increase by more than 27%, the equivalent of about 6.6 extra hours spent in range.
Follow-up data from the trial that were presented in February suggested that those results are long-lasting: Between six months and one years after switching to Medtronic’s system, the participants’ average glucose levels climbed only about 0.1%, while their time in range fell by about 0.7%.