Medtronic has secured the first FDA green light for a pulsed field ablation system, bringing a new type of atrial fibrillation treatment to the U.S.
By using short bursts of specifically tuned electric energy, pulsed field ablation disrupts certain types of cells more than others without generating excess heat. In cardiac ablation, that means taking aim at the cells in the heart muscle that cause irregular heartbeats—while sparing nearby tissues, such as the esophagus, from the damage that may come from the traditionally more indiscriminate methods of thermal ablation.
The FDA’s approval for the company’s PulseSelect system—for isolating the pulmonary vein in patients with intermittent or persistent afib—follows a previous breakthrough designation from the agency. It also comes shortly after a CE mark cleared the device’s use in Europe late last month.
“Launching the first FDA-approved PFA technology is not just a milestone; the PulseSelect PFA system is setting a new standard in safety for AF ablation with excellent efficacy and efficiency,” Rebecca Seidel, president of Medtronic’s cardiac ablation business, said in a statement. “It's a major step towards fulfilling our vision of providing disruptive electrophysiology solutions for patients.”
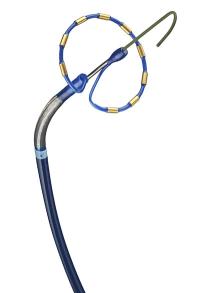
According to the company, PulseSelect has been designed as a plug-and-play device that can work with any fluoroscopy or heart-mapping system and that seamlessly takes the place of current catheters. In addition to ablation, the PulseSelect’s nine electrodes can also be used for cardiac pacing and sensing.
Medtronic said that commercialization of the PulseSelect system will begin in early 2024.
On the company’s heels is Johnson & Johnson’s Biosense Webster division, which announced the same day that it had begun enrolling and treating paroxysmal afib patients in a single-arm study evaluating its dual energy Thermocool Smarttouch catheter, capable of toggling between pulsed field ablation and traditional radiofrequency ablation.
Meanwhile, Boston Scientific put forward clinical data earlier this year from a study of its Farapulse system, showing it can operate on par with standard-of-care thermal and cyroablation therapies.