In the wake of earning FDA clearance for its insulin pump and automated dosing software—which connect to a Dexcom continuous glucose monitor to create what’s known as a hybrid closed-loop system for diabetes management—Beta Bionics is raking in the dough.
The Massachusetts-based company has pooled $100 million in series D equity funding, it announced this week.
Sands Capital and Omega Funds, both new investors in the company, co-led the financing, which was rounded out with participation from a mix of old and new backers: Marshall Wace, Soleus Capital, Eventide Asset Management, Farallon Capital, Perceptive Advisors, ArrowMark Partners, Pura Vida Investments and certain funds managed by RTW Investments.
The latest funding round easily tops Beta Bionics’ series C, which brought in $57 million in early 2022. It’s dwarfed, however, by the round before that, a two-part series B that raised nearly $130 million in two separate tranches in 2018 and 2019.
Beta Bionics will use the new backing not only to fuel the rollout of its newly cleared iLet artificial pancreas technology, but also to continue developing additional automated systems for people with diabetes.
“This significant investment represents a powerful vote of confidence in Beta Bionics' mission to redefine diabetes management with user-centric technologies,” CEO Sean Saint said in the company’s announcement. “We are eager to push the boundaries of what’s possible by expanding access to the iLet Bionic Pancreas nationwide and further develop and test the bi-hormonal bionic pancreas.”
The latter technology would use CGM readings to calculate doses of both insulin and glucagon—which is administered to help raise extremely low glucose levels—compared to the insulin-only iLet system.
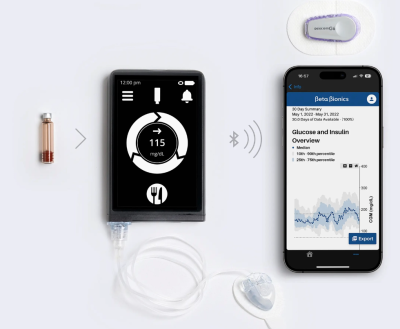
The iLet tech combines an insulin pump and automated dosing algorithm. The software collects and analyzes blood sugar readings from a Dexcom CGM, then uses those data to automatically adjust the insulin pump’s output based on the user’s real-time glucose needs.
While other closed-loop systems require doctors to program parameters into the dosing software, Beta Bionics’ “bionic pancreas” can get to work using only the user’s weight, hugely simplifying the setup process.
The company’s technology further pares down the learning curve associated with diabetes management and insulin dosing calculations by replacing the standard mealtime carbohydrate counts with a multiple-choice question: Users need only to estimate whether they’ll be consuming a small, medium or large amount of carbs.
In the FDA’s announcement about the system’s clearance in May, Jeff Shuren, M.D., director of the agency’s Center for Devices and Radiological Health, suggested those simplifications “will provide the Type 1 diabetes community with additional options and flexibilities for diabetes management and may help to broaden the reach of [automated insulin dosing] technology.”
Beta Bionics said at the time that the technology’s U.S. launch would begin “imminently.”