Novartis’ Alcon eye care division has withdrawn all versions of its CyPass micro-stent from the global market following new data from a long-term safety study. Used to relieve pressure under the eye following cataract surgeries for glaucoma patients, the company advised surgeons to stop implantations and return any unused devices.
The CyPass stent was first approved by the FDA in July 2016 after a two-year clinical study showed statistically significant reductions in intraocular pressure following the procedure compared to patients undergoing cataract surgery alone.
However, the extended, postmarket safety study showed that after five years, patients lost more endothelial cells—the cells lining the inner surface of the cornea, helping to maintain transparency—which was not seen at the two-year mark.
"We believe that withdrawing the CyPass Micro-Stent from the market is in patients' best interest and is the right thing to do," Stephen Lane, Alcon’s chief medical officer, said in a statement. Alcon first acquired the CyPass stent from Transcend Medical in February 2016.
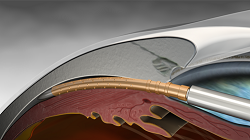
being implanted under the eye.
(Image: Transcend Medical)
Novartis previously announced plans to spin off Alcon in the first half of next year, with the new separately traded company being based in Switzerland and its current home base of Fort Worth, Texas, serving as a “critical location,” according to CEO Vas Narasimhan.
Novartis’ stock price seemed to weather the news, dipping only 2% compared to the previous day.
"Although we are removing the product from the market now out of an abundance of caution, we intend to partner with the FDA and other regulators to explore labeling changes that would support the reintroduction of the CyPass Micro-Stent in the future," said Lane. Alcon also said it will help surgeons with managing patients that have already received the stent.