In the wake of study results showing that its video-game-based treatment for symptoms of attention-deficit/hyperactivity disorder (ADHD) could be highly effective in adults—in addition to children aged 8 to 12, the only group for which the digital therapeutic is currently cleared by the FDA—Akili has plowed ahead in making the Endeavor program available for that older population.
People ages 18 and up with ADHD can now access a version of the therapy over the counter, without requiring a prescription, Akili announced Wednesday.
Though the company is still planning on submitting the adult-use program for official FDA clearance, it’s able to launch EndeavorOTC in the meantime due to a policy the FDA put in place amid the COVID-19 public health emergency that cleared a path for tech makers to roll out low-risk digital health tools for mental health treatment without going through the full regulatory review process.
EndeavorOTC is now available in the Apple App Store, where it can be downloaded onto an iPhone or iPad. Once downloaded, users can purchase a subscription plan, with prices starting around $10 per month. Akili said it will be regularly gathering feedback from users and using their responses to shape future versions of the digital therapeutic.
“Without reliable access to ADHD medication or sufficient mental healthcare professionals to meet demand, millions of Americans are in urgent need of new validated and accessible treatment options. Patients want better, and they want non-drug treatment options,” CEO Eddie Martucci said in Akili’s announcement.
“The core technology inside EndeavorOTC has helped thousands of children with ADHD, and recently has shown it dramatically helped adults in our clinical trial improve their focus, time management and organizational skills, and their ability to keep track of important items,” he continued.
Indeed, a recent study of the program showed a stronger impact on adults than had previously been demonstrated in children.
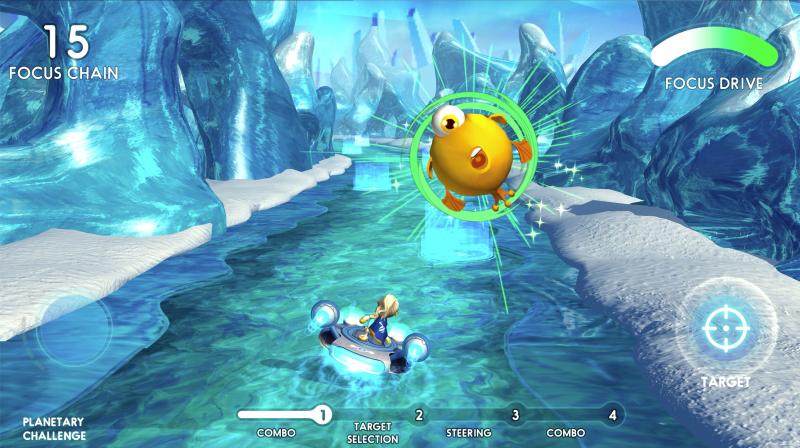
The treatment takes users through a multilevel interactive game that’s designed to stimulate the senses and challenge motor skills. Built-in algorithms constantly adjust the game in real time based on a user’s individual performance. According to the study, after using EndeavorOTC at home for six weeks, adult participants saw their attention levels improve by an average of nearly 6.5 points on the Test of Variables of Attention-Attention Comparison Score (TOVA-ACS).
Meanwhile, previous studies had found that the game resulted in TOVA-ACS improvements of about 2.6 points in teenagers and just under one point in 8- to 12-year-olds—the latter of which was enough of an improvement to earn the system its first FDA clearance.
Similarly, while more than 32% of the adults experienced at least a 30% reduction in their overall ADHD symptoms according to the ADHD Rating Scale-5, only 27% of teens and 24% of the younger group reached that threshold.
The launch of EndeavorOTC comes a few months after Akili announced that it would be narrowing its scope to focus only on building out its ADHD-focused digital therapeutics—in contrast to previous plans that included software to help treat lupus, depression, autism, multiple sclerosis and more.
That slimdown was sparked by Akili’s plummeting stock price and the sizable gap between its revenues and operating expenses—which totaled $323,000 and $90.6 million, respectively, for all of 2022. In addition to the pipeline reductions, Akili also announced at the start of this year that it would lay off 46 workers, comprising about 30% of its workforce, to help with the cost-cutting.
Editor's note: This story was updated to clarify the pricing of EndeavorOTC.