of Rotation Medical
The initial Series B round brought in $21.7 million back in July of 2014.
Existing investors New Enterprise Associates, Life Sciences Partners and Pappas Ventures participated in the financing. The financing is fundable in two tranches, the first being $8 million and the $4 million extension coming in 2017.
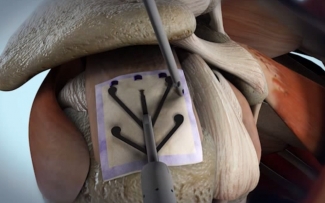
The Rotation Medical rotator cuff system uses a proprietary bioinductive implant and disposable instruments to perform the arthroscopic procedure quickly and easily.
“Since launching our Rotation Medical rotator cuff system in fall 2014, we’ve experienced rapid growth and very positive momentum,” said Martha Shadan, president and CEO of Rotation Medical, in the announcement. “This additional investment in our company will allow us to expand our U.S. presence and continue to support the tremendous physician adoption we have experienced thus far. We are very encouraged by physician and patient response to our bioinductive implant, which we believe serves an important role in helping people with rotator cuff disease heal and get back to an active lifestyle sooner.”
The FDA approved the bioinductive implant in March of 2014. The tech promotes new tissue growth at the site of implantation, increasing tendon thickness and healing tendon defects. This counters traditional treatment, which often can’t address tendon tissue. Tendons can further degenerate or re-tear after traditional treatments.
- here's the release
Related Articles:
Rotation Medical gets $27.2M to fund rotator cuff bioimplant launch
S&N backs OrthoSpace in $8M for pivotal U.S. trial of rotator cuff repair tech