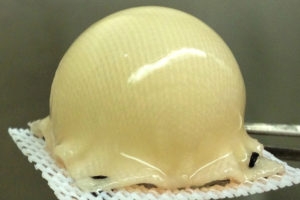
into the shape of a hip joint and covered with
cartilage made from stem cells.--Courtesy of
Washington University School of Medicine
in St. Louis
Researchers from the Washington University School of Medicine in St. Louis and Cytex Therapeutics have found a way to program stem cells to grow new cartilage on a 3-D template of the ball of a hip joint. This cartilage releases anti-inflammatory molecules that help fend off new occurrences of arthritis.
This new technology could result in an alternative to traditional hip replacement surgery and could eliminate the need for joint replacement surgery in some patients.
“We’ve developed a way to resurface an arthritic joint using a patient’s own stem cells to grow new cartilage, combined with gene therapy to release anti-inflammatory molecules to keep arthritis at bay,” said Farshid Guilak, professor of orthopedic surgery at Washington University, in a statement. “Our hope is to prevent, or at least delay, a standard metal and plastic prosthetic joint replacement.”
The cartilage is made with the patient's own stem cells taken from fat beneath the skin. A 3-D, biodegradable synthetic scaffold is molded into the shape of the patient’s joint. It is then covered with the cartilage and implanted onto the surface of an arthritic hip. This process helps alleviate arthritic pain and could delay or eliminate the need for hip replacement surgery.
Using gene therapy, developers were able to place anti-inflammatory molecules in the hip to fend off the reoccurrence of arthritis. As inflammatory molecules rise, cartilage in joints can be destroyed and pain increases, which this gene therapy can help deter.
“When there is inflammation, we can give a patient a simple drug, which activates the gene we’ve implanted, to lower inflammation in the joint,” said Guilak, who is also a professor of developmental biology and of biomedical engineering. “We can stop giving the drug at any time, which turns off the gene.”
The scaffold is built using a weave of approximately 600 biodegradable fiber bundles. The weaving pattern gives the scaffold the structure and properties you would find in normal cartilage. The implants are able to withstand a load of up to 10 times a patient’s body weight, Franklin Moutos, VP of technology development at Cytex, said.
Some customized implants using this stem cell-based tissue are already being tested in lab animals. Should all go well, we could see some devices ready for human testing in three to 5 years.
- here is the report
Related Articles:
Stryker gets FDA clearance to use Mako robot for hip replacement
Hospitals to shift CMS hip, knee implant cost pressure to medical device makers: Report
After tough times, hip implant revenue projected to grow again