Though the current conversation about brain-computer interfaces is largely dominated by two companies—Synchron, with a minimally invasive implant already in human trials, and Elon Musk’s Neuralink, which has faced a series of setbacks in its own race to the clinic—they’re not the only ones developing implants aimed at decoding neurological signals.
Paradromics, for one, is also building a device that it says could one day help restore communication to people who aren’t able to speak or type. The Connexus Direct Data Interface (DDI) technology has already caught the FDA’s eye: Like Synchron and Neuralink before it, the Texas-based startup announced Thursday that its neurotech has earned the agency’s breakthrough-device designation.
The tag clears away some of the obstacles on a novel device’s path toward FDA clearance. Not only will it push Paradromics’ future regulatory submissions to the top of the pile, it also opens up a direct line of communication between the company and the agency.
Paradromics’ Thursday announcement also included the news that it has closed a series A funding round. Totaling $33 million, the round was led by Prime Movers Lab—which previously led the startup’s $20 million seed funding in 2021—with additional backing from Westcott Investment Group, Dolby Family Ventures and Green Sands Equity.
The fresh infusion of venture capital will support Paradromics’ plans to begin clinical trials of its Connexus DDI brain-computer interface, which are reportedly slated to kick off sometime in the first half of next year, pending an FDA green light.
CEO Matt Angle, Ph.D., said in the release that the series A round “validates our leadership position among the small group of BCI platform companies on the verge of commercialization.”
“I think it’s no longer a question of whether brain computer interfaces will become the standard treatment for many neurological problems. It’s now about how big the brain-computer interface market will be,” said Dakin Sloss, founder and general partner at Prime Movers Lab and a member of Paradromics’ board of directors. “We’re seeing only a couple of companies emerge as real contenders in the space, and I believe Paradromics will be the one that moves into successful human trials.”
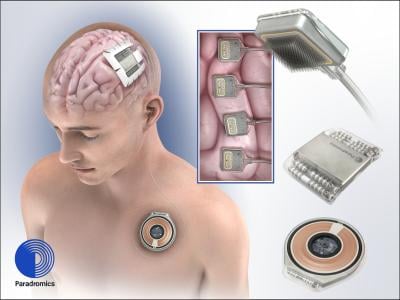
The cranial hub of the Connexus DDI system is implanted to sit flush with the surface of the skull. It features an array of microelectrodes that reach 1.5 millimeters below the surface of the brain to pick up signals from more than 1,600 neurons, according to Paradromics.
As the electrodes gather neurological activity, the cranial hub sends those data to a transmitter that’s placed under the skin on the upper chest. The transmitter, in turn, wirelessly passes on the brain readings to a connected computer, where—in the technology’s first application—they’re translated in real time to words and cursor movements, allowing it to be used as an assistive communication device.
According to the company, future applications could see the Connexus DDI system used to tap into the parts of the brain responsible for sensation and restore sensory deficits and to detect and treat mental illnesses, among other possible uses.